The process of sterilizing surgical instruments is a relatively young one, having been around for only about 100 years. In that time sterilization technology has however, developed quite quickly. The driving force behind this has been the common law requirement of a duty of care, leading to standards such as AS/NZS 4187: 2014 and NSQHS
Along with the maturing of these standards has come the requirement to more effectively trace surgical instrument through the sterilization process to their end use on a patient.
TABLE OF CONTENTS
Download our Free eBook

The Definitive Guide to Surgical Instrument Tracking
Part 1: The Evolution of Tracking
Just fill out the form and we will send it to you now
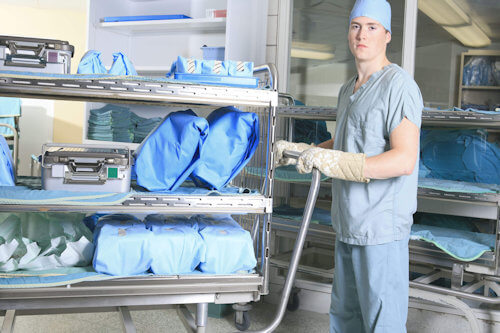
Under law, it is a requirement that all re-usable medical devices (RMD's) used on a patient are sterile.
In broad terms a surgical instrument tracking system provides proof of process. If for example, a patient was to report to a medical facility with an infection after having a medical procedure, it is incumbent on the medical facility to provide evidence of the instruments having went through a validated sterilization process.
A Surgical Instrument tracking System documents this process, allowing the medical facility to provide this evidence.
The advances in surgical procedures and surgical instruments over the last 20 years, have increased the complexity of RMD's that CSSD now re-process. This complexity has created new types of Sterilizers and Sterilizer Cycles.
As RMD complexity has evolved, infection control standards have had to adapt to the changes to take into account the cleaning and sterilizing of complex RMD designs.
Instrument tracking too has had to evolve in order to track these complex devices that require regular inspection and service.
This evolution begun with rudimentary tracking systems that utilised pen and paper and from paper it moved to batch tracking systems that utilised labelling guns and sticker books.
Now we have modern electronic systems that can individually track each surgical device through every reprocessing step from setup and packing, sterilization, storage, usage on a patient, return to CSSD and Decontamination.
These systems utilise a paper form designed to capture what instruments went into a sterilizer on a given date. The Sterilizing technician would fill out the form with their name, the date, the sterilizer number and cycle number followed by a hand written list of the items going onto the sterilizer.
Some of the positives of this type of system:
- Cheap
- Marginally better than nothing?
Some of the negatives of this type of system:
- The Technician can omit items in the list or write them incorrectly
- Legibility of what is recorded - hand writing can be difficult to read
- No tracking past the Sterilizer - No tracking to the patient - No Tracking through the Washers
- Storage of forms - Forms need to be kept in folders and archive boxes
- Retrieval of forms - a manual and very time consuming exercise.
- Recall is inefficient and time consuming
- Manual storage and retrieval of Check Lists
- Reporting needs to be manually assembled and is very time consuming


These systems utilise a a roll of label stickers inserted into a gun type applicator. The technician manually sets the output to be printed on the label by turning a number on dials on the gun. The labelling gun dispenses stickers that contains the Sterilizer number, the Cycle number and the date.
Two stickers are stuck to the outside of the packaging and one or more stickers are stuck to various pages in a record keeping book. One sticker is attached to the patient record.
Some of the positives of this type of system are:
- Costs less than Electronic tracking
- Marginally better than pen and paper systems
Some of the negatives of this type of system are:
- Record books still require Technicians to hand write the names of Trays and Singles
- The Technician can omit items in the lists or write them incorrectly
- Legibility of what is recorded - hand writing can be difficult to read
- Batch Tracking only - Does not track to instrument level
- Storage of record books - Books need to be kept in folders and archive boxes
- Retrieval of records - a manual and very time consuming exercise.
- Manual storage and retrieval of Check Lists
- Recall is inefficient and time consuming
- Reporting needs to be manually assembled and is very time consuming

Typically, an electronic system utilises barcode labels and scanners. Barcode labels representing instruments and trays are printed from a barcode label printer. The barcode labels are attached to the packs containing the instruments.
Barcode labels that identify staff and the sterilizer are also created from the system.
In Simple terms the barcodes on the instrument packs are scanned by the instrument technician, who then scans a barcode that represent a sterilizer, and finally scans a barcode that represents themselves.
As the barcodes are scanned, timestamps are taken by the tracking system and provide the name of the technician, the name of the instruments and the name of the Sterilizer that instruments were sterilized in and the date and time it all happened.
Some of the positives of this type of system are:
- Standards compliance
- Staff accountability
- Extremely accurate
- Instant retrieval of records
- Tracks though all processes; setup and packing, sterilization, storage, usage on a patient, return to CSSD and Decontamination.
- Compatible with all types of reprocessing
- Instant feedback on non complaint processes
- Efficient recall processes
- Instant reporting
- Self auditing - everything that is done is tracked
- Little or no handwriting
Some of the negatives of this type of system are:
- Cost of ownership. i.e. purchasing barcode scanners, label printers, computer workstations, server licensing etc.
- You need a plan B if your network systems go down. Some Tracking systems however, will have a manual fall back system that you can revert to in case of network down time.
